Research
Urinary Tract Infection
In our interdisciplinary research program, we have combined clinical and basic science approaches to detail a highly complex acute pathogenic cycle for uropathogenic Escherichia coli (UPEC), the most common causative agent of UTIs and the host response to infection. Our work has led to an increased understanding of UTIs including molecular mechanisms of susceptibility, establishment and progression of disease, epidemiology and determination of factors that influence disease outcomes and sequelae.
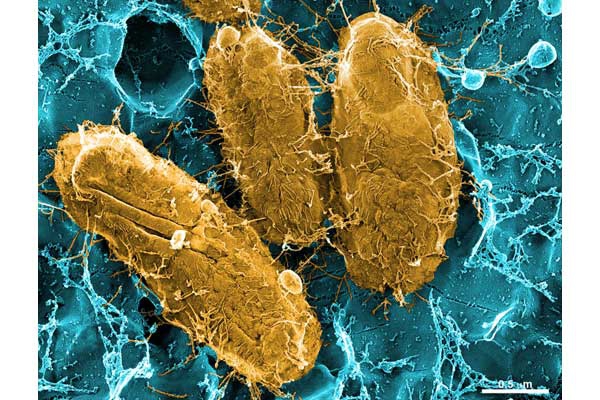
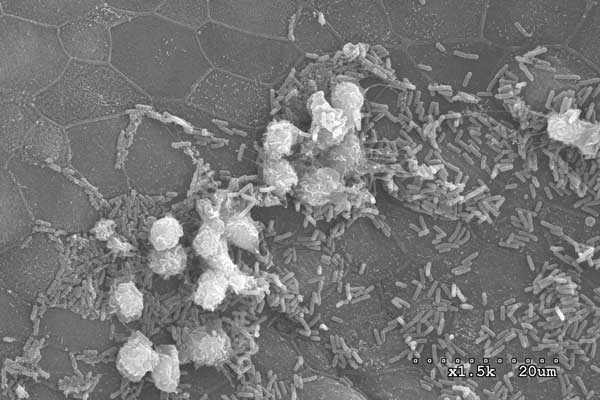
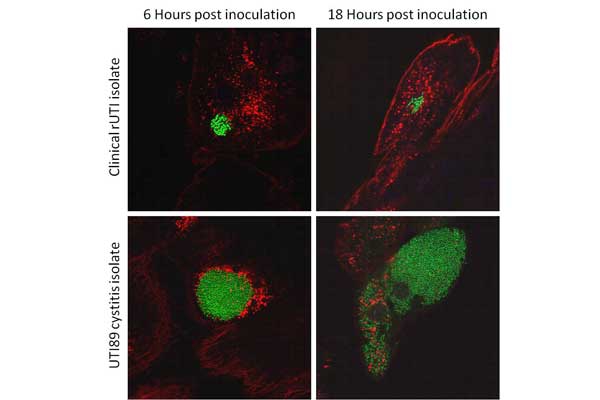
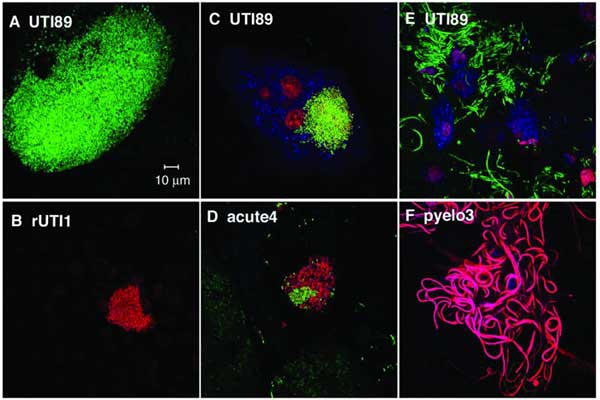
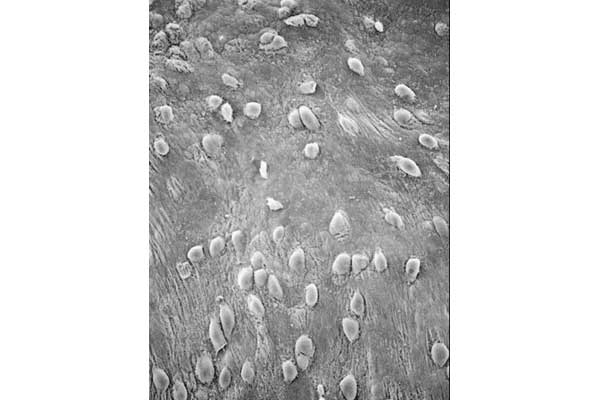
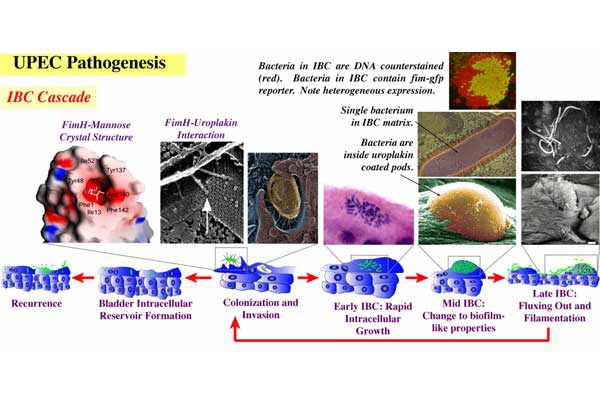
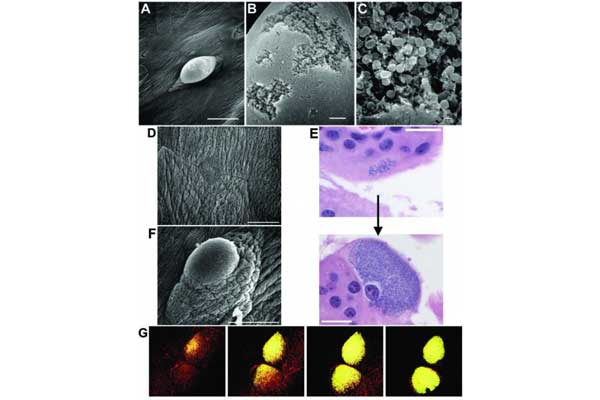
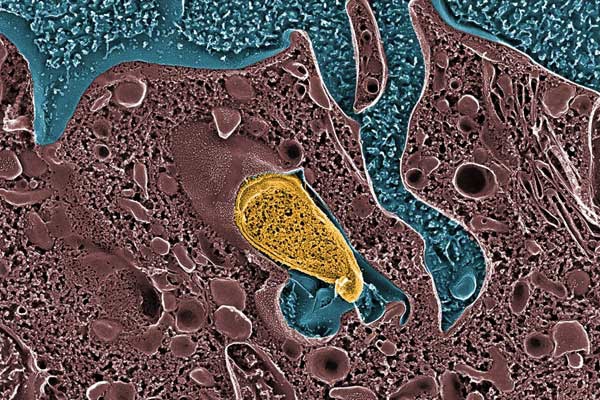
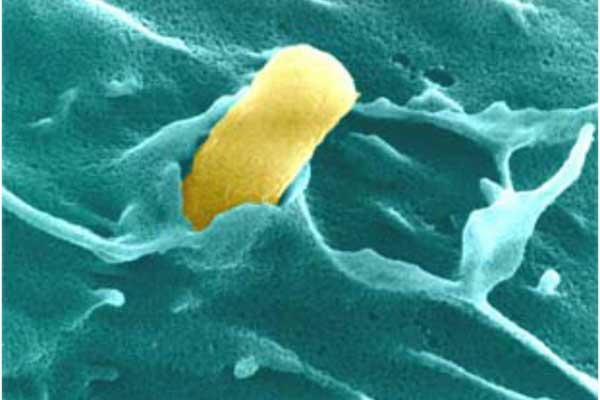
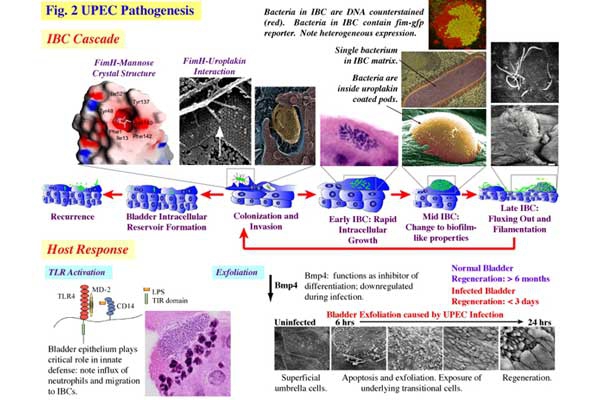
UPEC Acute Pathogenesis: Using our mouse model of urinary tract infection, we have delineated an acute pathogenic cascade that initiates when UPEC enter the mammalian bladder. Using type 1 pili with its adhesin, FimH, UPEC adhere to mannosylated residues on the bladder surface and invade into the superficial epithelial cells of the bladder. UPEC then enter the cytoplasm and rapidly replicate to form large intracellular bacterial communities (IBCs), with >104 bacteria per mature IBC within a few hours of invasion. IBCs are transient in nature. Once they mature, bacteria detach from the IBC and flux out of the host cell and go on to infect new host cells, repeating the IBC cycle. This pathway allows UPEC to gain a foothold in the host, building up in numbers in an intracellular niche protected from much of the host immune response. Further, UPEC filamentation upon fluxing from the IBC allows UPEC to resist the action of neutrophils in the bladder lumen. We have found that this cycle is critical for establishment of infection in naive animals. In addition, we assembled and used a large repository of UPEC strains to demonstrate that the ability to form IBCs is a general trait of UPEC. We have also shown that Klebsiella UTI is dependent on FimH mediated colonization and IBC formation. We have also verified in a human clinical study that IBCs and filamentous bacteria are observed in the urine of women with UPEC UTI, but not in controls or women with Gram-positive bacterial UTIs. Subsequently, multiple groups have reported that IBCs are present in the urine of UTI patients (including pediatric) and predict a more severe bout of infection. Currently, work determining bacterial mechanisms important in promoting the IBC cycle and long-term colonization of the bladder by UPEC are elucidating important aspects of disease progression and potential therapeutic targets. Interestingly, in contrast to the in vivo model, during in vitro infection of cell lines, the UPEC remains trapped within the endocytic vesicles. The factors responsible for this inability to recapitulate IBC formation in vitro are not yet understood. Consequently, this has hindered our ability to study the detailed molecular interplay during UPEC endosomal escape as well as IBC cycle. We are investigating the mechanism of UPEC intra-host growth in in vitro infection model by using primary bladder cells and manipulating the host signaling pathways.
Host Response: The host response to UPEC infection is a complex process that involves exfoliation of epithelial cells, release of cytokines and chemokines and the influx of inflammatory cells into the bladder. Bladder epithelial cells, which normally turn over only once in ~6 months, can be shed upon infection and regenerated within ~48hrs. This process involves TLR4 and cell death pathways within the superficial facet cells of the bladder, which are triggered by infection, and an upregulation of differentiation pathways in the underlying transitional epithelial cells. The resultant shedding of infected superficial facet cells is an important defense to expel bacteria from the bladder in the urine stream. However, this shedding also exposes underlying epithelial cells to infection. Further, upregulation of cyclooxygenase 2 (COX2) expression in urothelial cells and neutrophils in response to UTI is associated with neutrophil transmigration across the bladder epithelium. These COX2-dependent host responses are crucial for the associated mucosal damage that ensues during infection. Following this transmigration in mouse models of infection, persistent high-titer bacteriuria accompanied by severe immunopathology and ablation of the terminally differentiated superficial umbrella cells can develop in a syndrome we refer to as chronic cystitis that can result in a remodeling of the bladder tissue and a susceptibility to further recurrent UTIs even with less pathogenic bacteria. Elaboration of pro-inflammatory molecules such as interleukin 6 (IL-6) and granulocyte chemotactic cytokines such as IL-8; as well as neutrophils are observed in human urine during acute UTI and in our murine models. We are currently further delineating the epithelial response to infection and correlations between the levels of host response to infection and the outcome of infection. In addition we are investigating the role that differing gut microbiomes have on the host response to infection of the urinary tract. These studies are elucidating potentially important avenues of study for clinical susceptibility to deleterious disease outcomes, such as chronic infection and frequent recurrent UTIs.
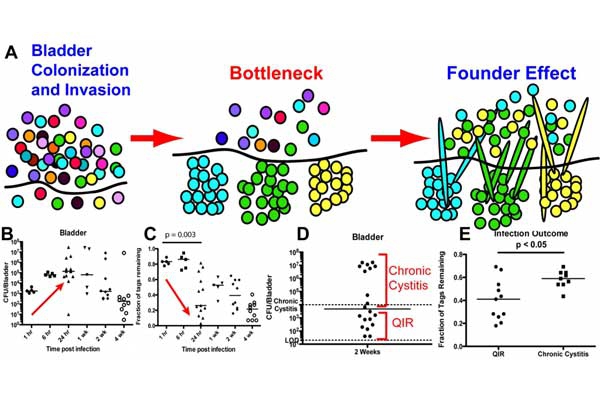
Recurrent and Chronic UTI: Reflective of different disease manifestations in humans, the outcome of experimental UPEC infection differs among inbred mouse strains. We find, in C57BL/6 mice, that quiescent intracellular reservoirs (QIRs) form in the bladder within 7-10 days of acute infection. QIRs are distinct from IBCs and are comprised of fewer than 15 bacteria persisting in a membrane-bound dormant state within transitional epithelial cells. QIRs are capable of emerging from dormancy to cause recurrent infections and bacteriuria and may represent one mechanism for recurrent UTI (rUTI) in humans. Using urines and periurethral swabs collected daily from women prone to rUTI, we identified colonization patterns consistent with reemergence of E. coli from bladder and gut reservoirs.
In contrast to C57BL/6 mice, C3H and CBA background mouse strains upon single inoculation of bacteria are susceptible to long-lasting, chronic cystitis characterized by persistent, high titer bacteriuria (>104 colony forming units (CFU)/ml), as well as high titer bacterial bladder burdens at sacrifice >4 weeks post-infection (wpi), chronic inflammation, and urothelial necrosis. Severe pyuria and elevated serum levels of IL-6, G-CSF, KC, and IL-5 within the first 24 hours post infection (hpi) predict with high certainty that chronic infection will develop. These events may reflect, in part, the natural course of cystitis in women, as placebo-controlled studies demonstrate that a majority of women remain bacteriuric for weeks after an acute episode of cystitis if not treated with antibiotics, despite overall improvement of symptoms. Further, in C3H/HeN mice, we found that a history of chronic cystitis is a significant risk factor for a subsequent recurrence of severe chronic cystitis. Understanding how prior infection influences a new infection is needed in order to understand clinical recurrent UTI. Thus, although it has long been known that a history of UTIs is the greatest independent risk factor for the development of a UTI, our recent studies elucidating the long-term consequences to the bladder tissue have shown that an initial chronic cystitis can remodel the bladder tissue, thus making it more susceptible for recurrent infections even with less virulent pathogens.
We developed a mouse model of recurrent UTI (rUTI) in which we allowed an initial UPEC infection to either self-resolve or develop into chronic cystitis lasting two weeks or longer before treatment with antibiotics and a period of convalescence and then challenged the mice with a second inoculation of UPEC. We found that the outcome of the initial infection was strongly correlated with the response of the mouse to the second bacterial challenge. Mice that spontaneously resolved the first infection (resolved mice) were resistant to subsequent UTI, whereas mice that had previously progressed to chronic cystitis (sensitized mice) were more susceptible to severe recurrent UTI, even if the challenge strain differed from the initial sensitizing strain. Examination of the bladder epithelium revealed that the superficial umbrella cells of sensitized mice were smaller than those from resolved mice or age-matched adult naïve controls and lacked markers of terminal differentiation. Furthermore, proteomic and transcriptomic analyses revealed that infection can leave a molecular imprint on the bladder, which can predispose to rUTI, even after clearance of the infection with antibiotic therapy. Susceptibility to rUTI was strongly correlated with the expression of cyclooxygenase-2 (COX-2) following challenge infection and could be reduced by treatment with a selective COX-2 inhibitor. This is consistent with previous observations showing that activation of a host-pathogen checkpoint influences the outcome of infection. In addition, we found that the dynamics of TNF signaling activation differed when the initial infection was chronic (sensitized) compared with self-limiting (resolved). Inoculation of mice who had experienced a prior bladder infection with UPEC resulted in an earlier onset of TNF-mediated inflammation compared with age-matched naïve mice. This early-phase TNF signaling was shown to decrease colonization by facilitating rapid recruitment of neutrophils and exfoliation of infected bladder cells. By contrast, mice that suffered from chronic UTI following initial infection experienced a prolonged TNF signaling period, which exacerbated inflammation and worsened infection. Thus, the regulation of TNF dynamics and COX-2 expression could explain how a prior infection can modulate susceptibility to future infections.
UPEC colonization of the gastrointestinal tract (GIT): A healthy gut microbiota maintains homeostasis with the host immune system and prevents colonization by bacterial pathogens. Ironically, antibiotic treatments intended to clear bacterial infections often induce dysbiosis of the microbiota in both mice and humans, thus exposing individuals to an increased risk of colonization or expansion of potential pathogens in the gut including UPEC. UPEC commonly exists as a minor member of the gut microbiota, establishing a reservoir in the gastrointestinal tract (GIT) from which UPEC can then be shed in the feces and colonize the periurethral area or vagina. UPEC can subsequently ascend through the urethra to the urinary tract (UT) to cause infection. At the time of a UTI, the causal E. coli strain is most often the predominant E. coli strain in the feces and could be identified on patient periurethral swabs several days prior to onset of UTI symptoms, indicating a GIT to UT route of infection. Despite appropriate antibiotic treatment, more than 60% of recurrent UTIs (rUTIS) are due to the same strain of E. coli that caused the initial infection, consistent with re-inoculation of the UT from a persistent GIT reservoir. UPEC persistence within the human gut microbiota is an important susceptibility factor for rUTI.
Numerous studies have shown that one function of a healthy microbiota in humans is to resist colonization (described as colonization resistance (CR)) by pathogens, which can be affected by antibiotic treatments. Loss of CR following antibiotic treatment is modeled in mice by oral administration of the antibiotic streptomycin (STM), which potentiates intestinal colonization of bacteria, like Salmonella and various commensal E. coli strains. The lab has demonstrated that a single dose of STM by oral gavage reduces this CR function in conventional mice, allowing the invasion of the cystitis UPEC isolate, UTI89, into the microbiota, where it is able to colonize and stably persist up to three weeks. While an STM-resistant, lab-adapted E. coli strain, MG1655, can colonize the GIT of mice receiving continuous STM treatment, a single STM treatment is insufficient for persistent colonization by MG1655. Further, we have found that type 1 pili and its adhesin, FimH, and F17-like pili and its adhesin, UclD, are critical for stable mouse GIT colonization. Further, we have used oral dosing with high affinity mannosides, which inhibit FimH function, to selectively deplete UPEC from the GIT of colonized mice without affecting the rest of the GIT microbiota. Studies to further elucidate the molecular basis by which UPEC forms a reservoir in the GIT and consequences of UPEC GIT colonization will lead to therapeutic development of treatments to reduce UPEC GIT carriage and thus reduce the risk of UPEC introduction into the UT and rUTIs.
Collaborators: Thomas “Mac” Hooton, Ann Stapleton, Marco Colonna, Ashlee Earl.